How to grow an organ with a 3D printer
Bioprinting is the process of synthesizing objects that comprise living cells and imitate natural tissue via layer-by-layer growth. This field is a result of the integration of technologies from bioengineering, materials science, molecular biology, and chemistry.
Bioprinters work much like ordinary 3D printers. The only difference is that instead of plastics, ceramics, or metals they use bio-ink – a slurry of cells, nutrients, and gels that emulate the extracellular matrix. This matrix, which consists of glycoproteins (like collagen), hyaluronic acid, minerals, and several other components, serves as a medium for the transfer of chemicals between cells and provides them with structural support.
A bioprinter creates an organic object by depositing the bio-ink layer-by-layer according to a digital model. These models are unique to each patient, made using MRI scans of their organs along with various other medical imaging techniques.
Biomaterials produced by 3D printing have numerous applications in tissue engineering and medicine in general, such as the synthesis of bones, skin, blood vessels, or other tissues and organs for the purposes of transplantation and grafting. This technology even has the potential to eliminate donor organ scarcity by making it possible to print hearts, kidneys and livers.
Unfortunately, so far it has been impossible to manufacture such complex organs, since their structure and functions are very hard to replicate via printing. For example, a heart must not only be structurally sound, but also be capable of vascularization (the process of forming new blood vessels in tissue), contraction, and propagation of electrical signals.
However, there has been some success when it comes to printing simpler organs. For instance, scientists from Wake Forest University in the US have engineered and successfully implanted artificial bladder tissues to seven patients suffering from various bladder diseases.
Another application of bioprinting is the use of plant cells to create artificial wood and other organic materials. This would help reduce deforestation and allow restoration of woodlands without depriving industries of their feedstock.
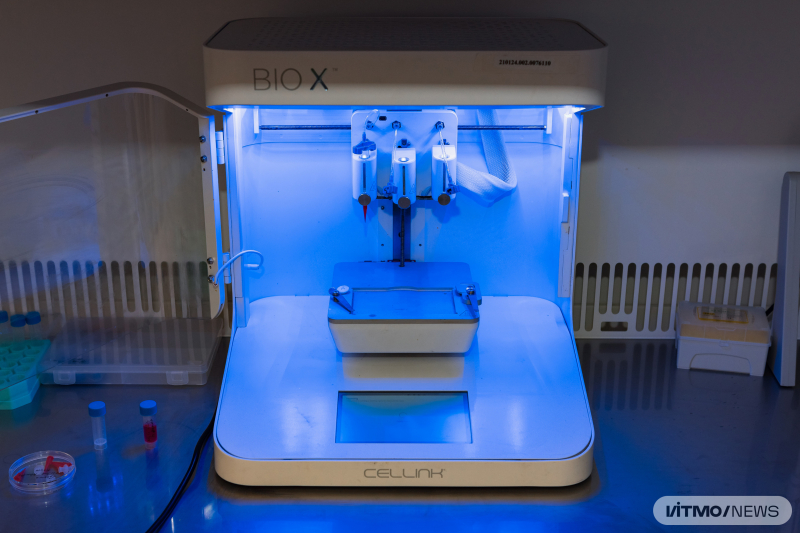
A 3D bioprinter at an ITMO University lab. Photo by Dmitry Grigoryev / ITMO.NEWS
Bioprinting at ITMO: regenerating cartilage with implants
In ITMO University, there are a few bioprinting-related projects in the works. Notably, the Laboratory for 3D Printing of Functional Nanomaterials has been supported by the Russian government with a megagrant to help develop the technology. It focuses on creating implants to regenerate damaged hyaline cartilage, which is a type of connective tissue that can be found in the joints, ribs, nose, larynx, and trachea.
Defects in hyaline cartilage may appear due to a variety of causes, such as injuries, physical activity, or obesity. While some types of damage can be repaired via non-invasive treatments, the more serious cases require surgical treatment, including chondroplasty (cartilage transplantation), and chondrocyte (cartilage cells) implantation. Yet these methods have significant shortcomings: it is not always possible to recreate a structure that is anatomically similar to the original, and the restored tissue may, over time, be eroded by its own enzymes that are foreign to the recipient’s body. One promising way of solving these issues is the use of cell engineering to produce hyaline cartilage on a large scale, which necessitates precise and streamlined 3D bioprinting technologies.
Viktoria Egorova, a student at the ChemBio Cluster, is responsible for the project, which will be included in her Master’s thesis. The initial idea belongs to Mikhail Bozhokin, a research associate at the R.R. Vreden Russian Scientific Research Institute of Traumatology and Orthopedics, who acts as a scientific advisor, while Dr. Elena Krivoshapkina, an associate professor at the ChemBio Cluster, serves as supervisor. Scholars from Saint Petersburg State University and the Institute of Cytology of the Russian Academy of Science also took part in the project.
“I am interested in printing with cells and seeing what happens when they are enveloped by a medium that imitates their natural state. Coincidentally, the biologist Mikhail Bozhokin, who specializes in casting cartilage implants and populating those casts with living cells, was visiting ITMO when he shared with Dr. Elena Krivoshapkina his idea for a bioprinting project. The goal is to automate the printing of implants so that research of their cells can be carried out on a large scale. After all, the bigger the data set, the more relevant it is, which makes it easier to gather statistical evidence and keep track of progress,” shares Viktoria Egorova, the project’s lead.

Victoria Egorova. Photo by Dmitry Grigoryev / ITMO.NEWS
A standardized culture of human cells, mixed with other organic matter, is used as bio-ink. This jelly-like substance is loaded into the extruder of a 3D printer, which makes biological objects by forcing the material (cells in this case) through a nozzle, thus shaping it. An engineered cellular construct of a given structure and accuracy emerges as a result. This technique is called extrusion printing.
What is particularly important is that the materials used alongside living cells have to be non-toxic, as the cells must be able to survive within the ink, during the extrusion process, and when further growing the manufactured object. These materials also have to fit certain criteria when it comes to their rigidity and viscosity so that they can later be utilized in non-invasive treatments.
Results
The project’s creators have already figured out how to make biomaterials with optimal properties and printed implants that cells can survive in. Such implants possess a framework of polymers containing a hydrogel that keeps cells alive. In order to achieve this, researchers combined two technologies: extrusion printing for the gel and LCD printing (a 3D printing method that uses LCD lights to harden light-sensitive materials into the desired shape – Ed.) for the framework. The resulting structure is then incubated, meaning cells are allowed to grow within it. If the cells survive, multiply, and form a tissue-like structure, they then produce collagen which increases the rigidity of the implant. That makes it suitable for use in surgery.
Moving forward, the team behind the project plans on conducting in vitro experiments to study the compatibility of their implant’s material with living cells and determine the optimal hydrogel properties (such as rigidity). In order to successfully repair a simulated hyaline cartilage defect, the scientists will first run tests on rabbits. Viktoria Egorova points out that while there were many attempts to use 3D printed implants to fix cartilage damage, no one has yet to streamline the technology and conduct animal testing.

A 3D bioprinter at an ITMO University lab. Photo by Dmitry Grigoryev / ITMO.NEWS
The researchers also use a special device from the R.R. Vreden Russian Scientific Research Institute of Traumatology and Orthopedics to conduct their experiments. It is capable of making a defect of any given size and shape inside the cartilage of a rabbit. The researchers then produce a matching 3D model that is used to bio-print an implant.
Future prospects
“The world is changing rapidly and I believe that it is important to stay flexible. As part of my Master’s thesis, I plan to conclude this research by achieving cell survivability and successful in vivo experiments in order to demonstrate that this approach will lead to further development of cell engineering technologies in Russia. In the future, I plan on continuing this project as part of my PhD studies, eventually developing an even more complex system of microfluids that would not just imitate the extracellular matrix, but aid in the metabolic activity of different cell cultures that it contains. I would also like to create a structure that functions similarly to organs-on-chips (devices used to grow various cell cultures),” says Viktoria Egorova.
The project’s creators predict that in the future, 3D bioimplants for hyaline cartilage repair will be used to restore damaged joints. As diseases of the musculoskeletal system, hyaline cartilage defects in major joints (such as the knee and hip) among them, raise more and more concern, this field of study seems very relevant. Joint damage is especially common among injured athletes and among the elderly affected by arthritis and arthrosis. Furthermore, 3D bioprinting opens up the possibility of personalized treatment – any defect can be fixed if its structure, shape, size, and other properties are known.

Victoria Egorova. Photo by Dmitry Grigoryev / ITMO.NEWS
“If our technology can be replicated, we would be able to patent it and make it available to medical organizations, research institutions, and private healthcare facilities,” sums up Viktoria Egorova.