Not all bubbles are good
Probably everyone has seen bubbles that form along the walls of a water bottle when it is left undisturbed for a while. This phenomenon is best observed in soda. The thing is, water is inhomogeneous, meaning that all liquids contain numerous impurities, mostly atmospheric gases.
That’s why under certain conditions, you can observe cavitation in liquid media. This process occurs when the liquid pressure changes and is characterized by the formation and bursts of bubbles. They can rapidly shrink and expand, while the temperature of the gas inside these bubbles fluctuates greatly and can reach several hundred degrees Celsius.
This effect is highly widespread and it can oftentimes have a serious negative impact. A vivid example is cavitation erosion in metals that can damage vital devices, such as ship propellers and hydraulic turbines. That’s why materials are gradually destroyed by cavitation when they are in constant contact with a liquid.
A ship propeller damaged by cavitation corrosion. Photo by Erik Axdahl / wikipedia.org (CC BY-SA 2.5)
Cavitation, however, has positive effects, too. This phenomenon is common in ultrasonic cleaning of solid surfaces. This is how it works: ultrasonic cleaners create cavitation using sound waves in liquids, then cavitation bubbles burst, thus generating shock waves that destroy pollutant particles or isolate them from the surface.
What’s more, some researchers have suggested that cavitation can be exploited in targeted drug delivery. Theoretically, specialists can make use of an ultrasound unit once an encapsulated drug is released into the bloodstream. They can place a device on a specific area to generate cavitation bubbles, which by popping will destroy a capsule with a drug and ensure it reaches the tumor area. For this to work, scientists need to know exactly how bubbles will interact with one another, with liquids, and with various solid barriers, as well as how the system will function in general. After all, the bursts of bubbles produce great amounts of energy and if anything goes wrong, the procedure may cause tissue damage or even be fatal. Yet if scientists figure out how to generate bubbles of certain sizes and resonant frequencies, these bubbles will pop just enough to destroy the pill capsule only.
Predicting the system
The same processes are currently studied by researchers from ITMO’s Infochemistry Scientific Center. Their recent study showed that the behavior of air bubbles varies in solutions with different concentrations. It also resulted in a specifically trained neural network that can record these changes and determine a solution’s concentration via its bubble image.
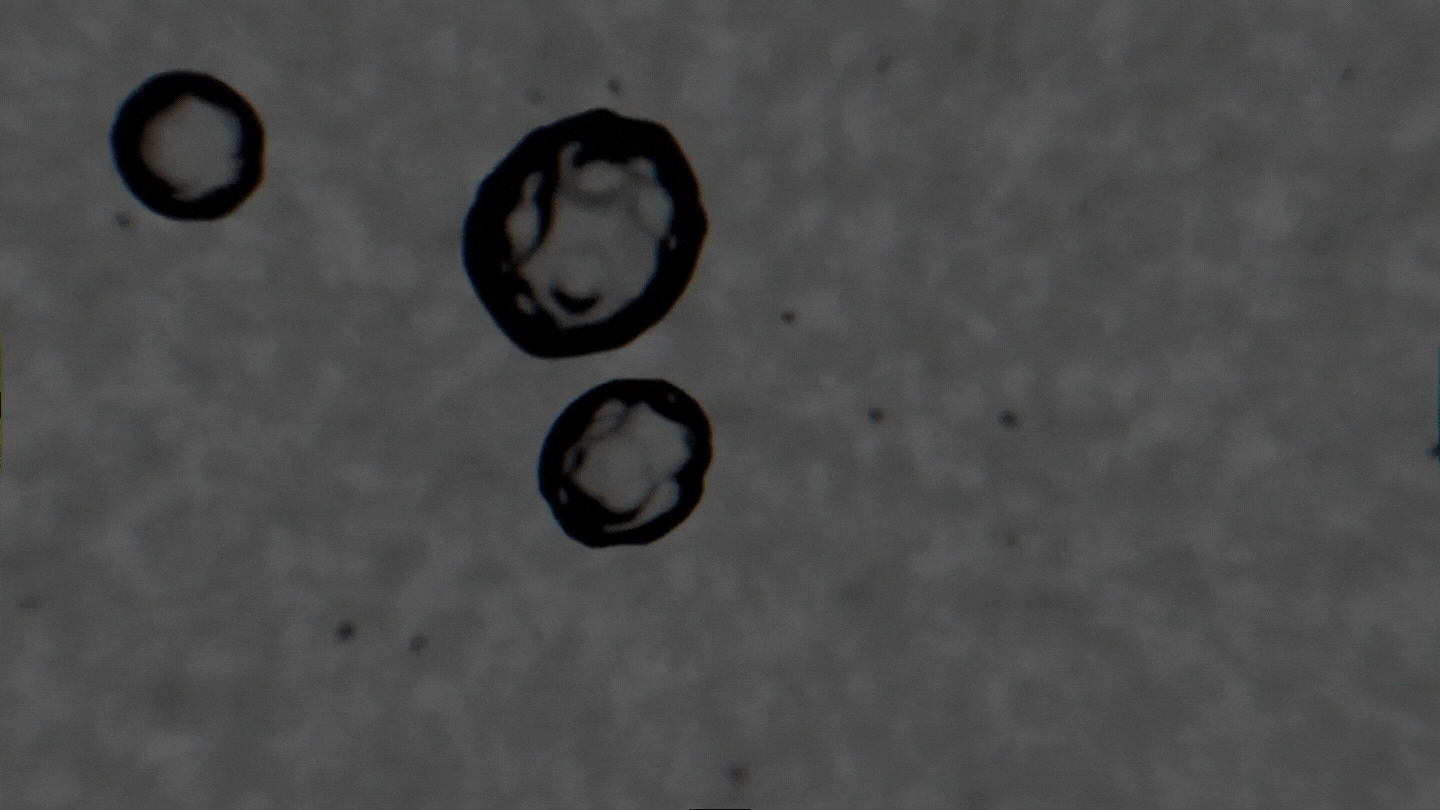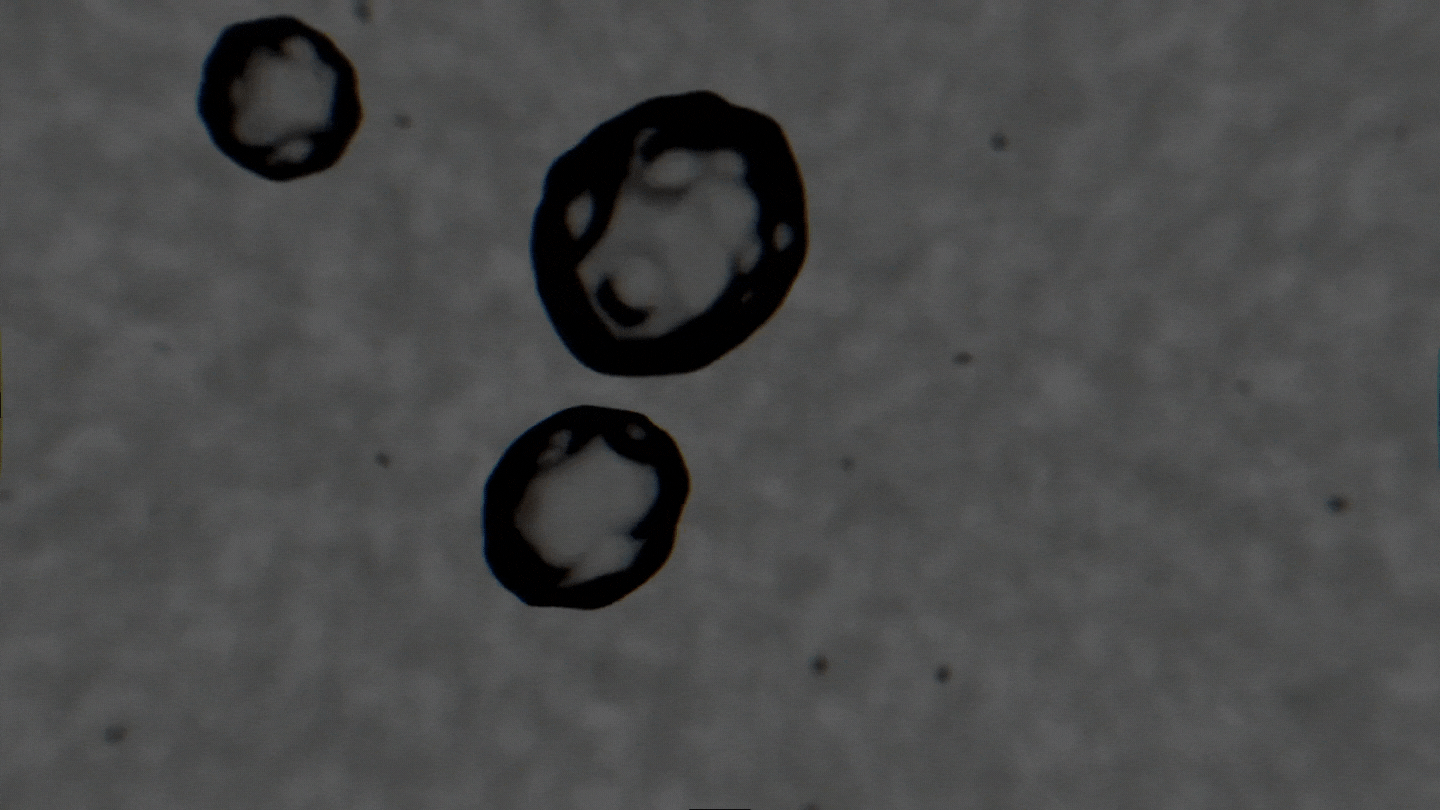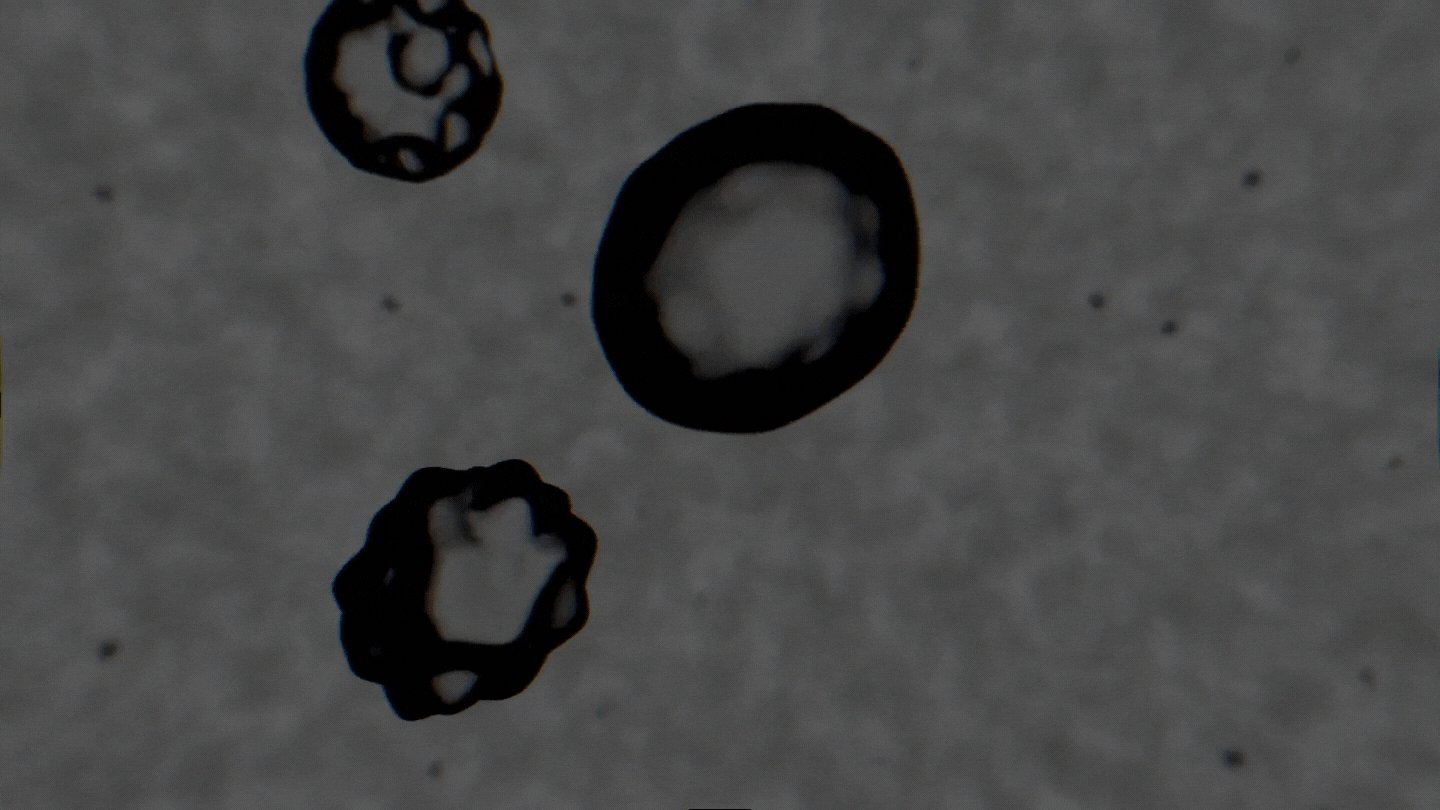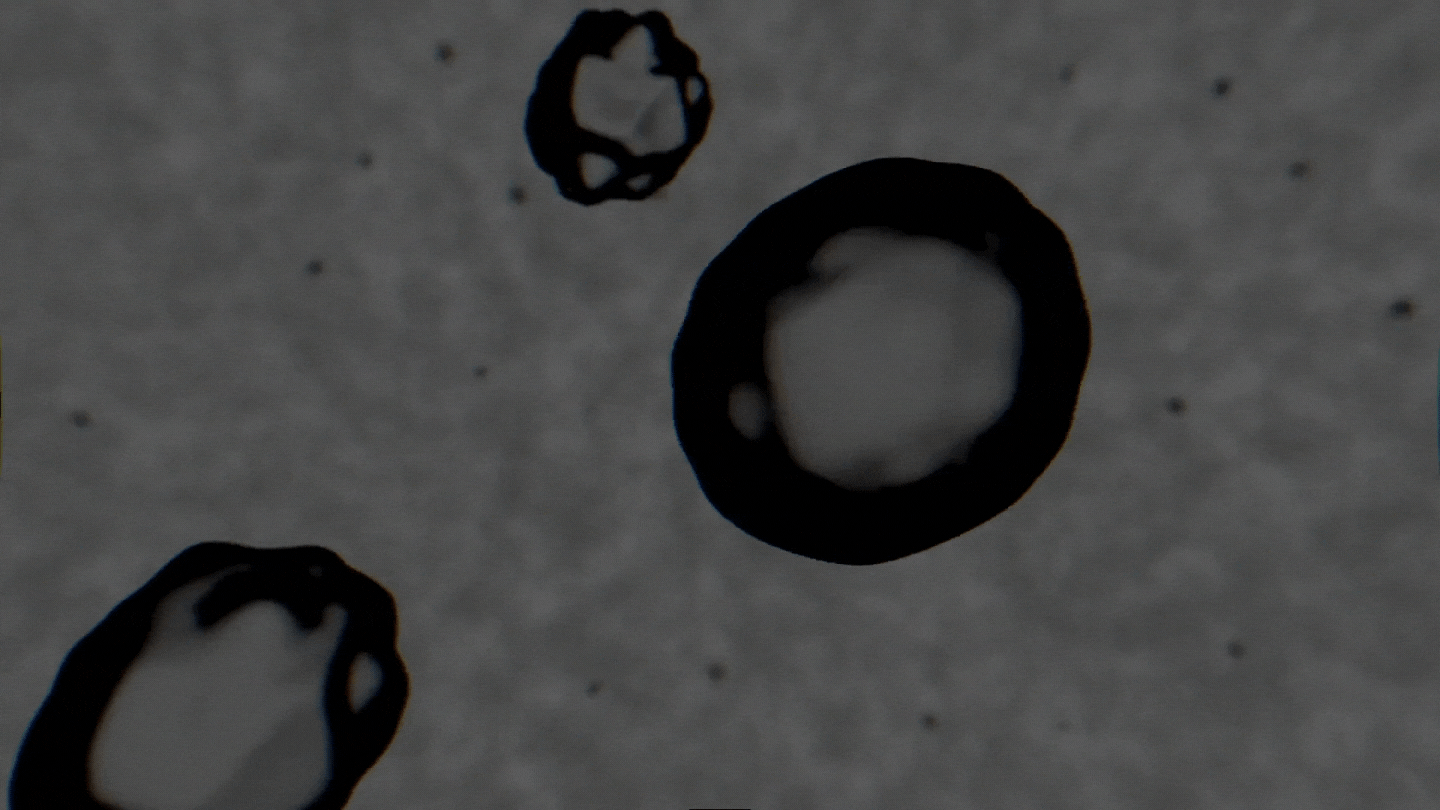
The formation of cavitation bubbles in a solution under the influence of ultrasound. Video courtesy of the authors
Air bubbles were observed using an experimental setup, which generated bubbles in a water-alcohol solution and recorded the dynamics of their oscillations with a high-speed camera. In their study, the scientists tried to create conditions as close to real ones as possible. Bubbles moved freely and were recorded as their travel through the medium. As a result, the researchers received a database with over 10,000 images, which were then used to train the neural network. The technology demonstrates the accuracy of 89% and can determine alcohol solutions with concentrations between 5% and 96%.
The study was first initiated by Ilya Korolev, an 11th grade student at School No. 358, who after his one-week internship at the center in 2020 decided to stay and continue his work. As he admits himself, his initial interest in studying the interaction of cavitation bubbles in various solutions evolved in a highly scientific work. The results of their research were published in an international scientific journal.
“When I first came to the center, I had no idea what I wanted to do. Somehow I came across an internship opening and decided to give it a try. During my one-week internship here, I worked side by side with lab mentors and did my own projects. But a week was not enough so I reached out to Ekaterina Skorb (the head of ITMO’s Infochemistry Scientific Center – Ed.) and she said that I can come on Sundays to discuss projects and my career plans. That’s how I got into the center and now I have my article published,” says Ilya Korolev. “Having worked with experienced researchers, I am now confident that I want to do science in the future. Surely, I’d love to work abroad and even have my own research team, and, of course, collaborate with various companies.”

Ilya Korolev. Photo courtesy of the subject
What’s next?
As noted by the authors, their study can be applied in various fields. By confirming that machine learning can be used to predict the dynamics of cavitation bubbles, the researchers paved the way for its more efficient use in targeted drug delivery and other fields.
The discovery may also find its application in oil refineries or food factories that require a strict control over the composition and concentration of raw materials in use.
“The neural network can help researchers analyze the compositions of complex mixtures, as well as detect small concentrations of impurities, an octane rating of gas, and so on. Representatives of Tatneft and Gazprom Neft became interested in our technology when they visited the laboratory,” comments Ekaterina Skorb, the head of ITMO’s Infochemistry Scientific Center.

Ekaterina Skorb. Photo by ITMO.NEWS
The results of the study will be made public to let anyone interested use their advances. In the future, ITMO’s Infochemistry Scientific Center may open its own repository, which will bring together a wide range of open-source solutions created by its specialists.
Reference: Ilya Korolev, Timur A. Aliev, Tetiana Orlova, Sviatlana A. Ulasevich, Michael Nosonovsky, and Ekaterina V. Skorb. «When Bubbles Are Not Spherical: Artificial Intelligence Analysis of Ultrasonic Cavitation Bubbles in Solutions of Varying Concentrations», Journal of Physical Chemistry, April 18, 2022.





