Battle for a place under the sun
Cancer is one of the world’s leading causes of death, with the WHO estimating that it took nearly 10 million lives in 2020. Though many research efforts are dedicated to developing new treatments for the majority of oncological diseases, early diagnostics is still the key factor in therapeutic success.
Among the available kinds of treatment, chemotherapy is one of the most common. During chemotherapy, chemical compounds introduced via pills, injections, or capsules destroy cancer cells.
For many of the widespread forms of cancer, chemotherapy is considered the primary course of treatment, as it has the best chances of keeping the disease at bay for longer periods of time. However, it comes with its side effects: the drugs used in chemotherapy have a body-wide effect (for instance, targeting all cells that have reached a certain stage of development), which means they damage both malignant and healthy cells alike. This leads to weight and hair loss, as well as dizziness, vomiting, fatigue, anemia, and other conditions.
According to Anton Muravev, an associate professor at ITMO’s Infochemistry Scientific Center, one solution that can help lower chemotherapy’s negative side effects is developing drugs with lower toxicity for healthy cell lines. Moreover, it’s important to find a way to overcome the defenses that cancer cells put up against apoptosis.
Apoptosis is a mechanism of programmed cell death; normally, its functions in the body include the timely replacement of old cells with new ones, thus preventing genetic mutations. However, if DNA mutations do occur, a cell can turn into a cancerous one and will resist apoptosis to a degree that renders it nearly immortal. That’s why one of the key objectives in cancer research is to produce a treatment that would destroy this apoptosis defense mechanism in cancer cells without endangering the healthy ones.
“Cancer cells are like weeds fighting with other plants for their place under the sun. If you don’t extract every one of their roots, then, in some time, they will grow again. It’s the same with cancer cells: they divide quickly, taking the place of healthy cells and protecting themselves from apoptosis. This makes them immortal. That’s why it’s important to develop compounds that can overcome these cells’ defenses and trigger their deaths. At the same time, such compounds have to affect only tumor cells and be harmless to healthy ones,” explains Anton Muravev.

Anton Muravev. Photo by Dmitry Griroyev / ITMO.NEWS
Molecular “grapes”
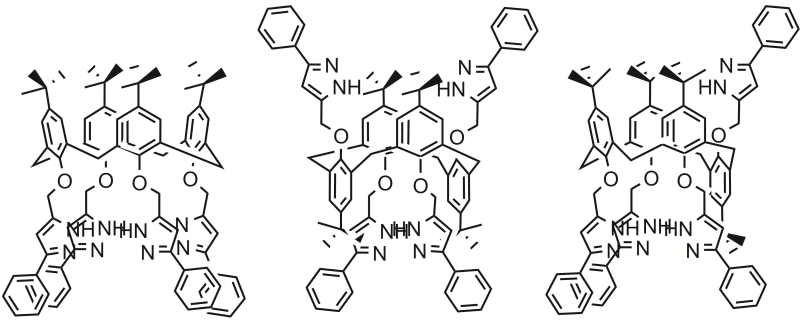
The development of such compounds was addressed by a team from ITMO’s Infochemistry Scientific Center and A.E. Arbuzov Institute of Organic and Physical Chemistry (part of the Kazan Scientific Center of Russian Academy of Sciences). The researchers synthesized antitumor compounds with different geometries and chemical and biological properties, producing nine different molecular structures in total.
Each structure is made of two parts and resembles a cup with grape clusters falling out of it. The “cups” themselves are non-toxic macrocycling calixarene molecules; they are made from linked chains of phenol and formaldehyde, which accounts for their shape. This kind of structure can be easily bound with other molecules, controlling their passage into the cell. The “grape clusters” attached to the cups are pyrazole molecules capable of binding to cell DNA, which can trigger apoptosis.
Anton Muravev shares that these compounds had been developed jointly by scientists from ITMO, A.E. Arbuzov Institute of Organic and Physical Chemistry, and University of East Anglia (the UK) for over five years.

A rotary evaporator used to remove the solvent from the final mixture. Photo by Dmitry Griroyev / ITMO.NEWS
How it works
The nine resulting compounds have been tested for anticancer effects. For this, they were applied to healthy cells from the liver and lungs, as well as cancer cells from the cervix, lungs, and prostate. Three compounds were proven most effective: they destroyed cancer cells from the cervix while maintaining the viability of healthy cells.
“At the end of the first stage of its lifecycle, a cell checks for any damage in its DNA. If any mistakes in a cell’s genetic information are identified, it releases special compounds meant to correct any mutations. If these compounds are unsuccessful, the cell triggers apoptosis. A cancer cell will resist this process – and that’s when our compounds come to the rescue. They interact with the cancer cell’s DNA, triggering apoptosis, which leads to the death of the cell,” explains Anton Muravev.
According to the researchers, the developed antitumor compounds have the following advantages:
Selectivity. They affect only cancer cells, leaving healthy ones intact. An additional experiment demonstrated that after 24 hours, apoptosis was triggered in cancer cells treated with the compounds, while healthy and dead cells remained unchanged. The results of the test were compared to the effects of doxorubicin, a popular cancer treatment that suppresses both cancerous and healthy cells.
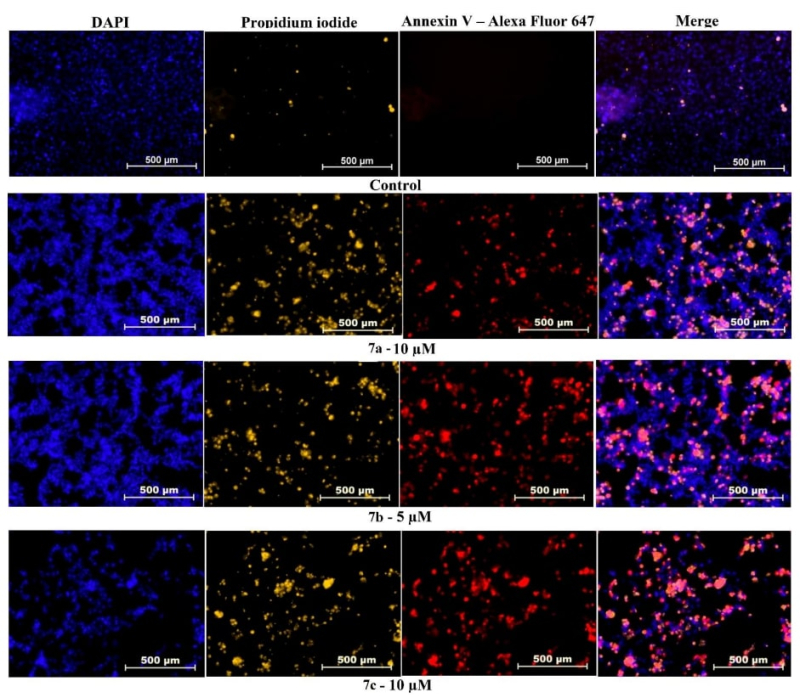
Experimental images depicting the apoptosis of cancer cells. The researchers compared the cervix cells pre-treatment (first row) with their condition after treatment with the new compounds (rows 2-4). Living cells are dyed blue, dead cells are dyed orange, and cells undergoing apoptosis are dyed red. Illustration from the article, credit: ScienceDirect
Low toxicity. They are safe for use in the human body, as proven in two experiments. The first one showed that anticancer compounds didn’t trigger hemolysis (the destruction of erythrocytes in the blood). In the second experiment, the compounds were injected into healthy mice to identify the lethal dose.
What’s next
Anton Muravev states that next on the group’s agenda is an in vivo test of the compounds – injecting them into mice with cancer and running tests on other kinds of tumor cell cultures. However, the key objective right now is to test the new compounds for mutagenicity – the capacity to cause mutations and side effects in healthy cells.
“According to our evaluations, the compounds don’t trigger mutations in DNA molecules. Now, we want to prove this data experimentally. This is an important step because we will see what happens in the body during prolonged chemotherapy and whether any side effects will manifest in the process,” concludes Anton Muravev.

Anton Muravev. Photo by Dmitry Griroyev / ITMO.NEWS
This research project was supported by the Russian Science Foundation (project No. 21-73-10185).
Reference: Anton Muravev, Alexandra Voloshina, Anastasia Sapunova, Farida Gabdrakhmanova, Oksana Lenina, Konstantin Petrov, Sergey Shityakov, Ekaterina Skorb, Svetlana Solovieva, Igor Antipin. Calix[4]arene–pyrazole conjugates as potential cancer therapeutics (Bioorganic Chemistry, 2023).