Although found in alloys, paints, electric motors, batteries, and polymers, cobalt and lead are among the most hazardous pollutants. They make water unusable and toxic, lead to plant death and reduce crop yields, and can cause poisoning and increase the risk of heart diseases in humans.
Industrial wastewater treatment plants employ a range of methods to remove heavy metals from water, but they all have their drawbacks. For one, a membrane-based treatment, despite its efficacy, is costly to manufacture and therefore to scale up. Chemical agents can cause a lot of sludge, a small powdery sedimentary deposit, which can re-pollute water. And, finally, photocatalysis is pH-dependent and efficient solely with organic pollutants.
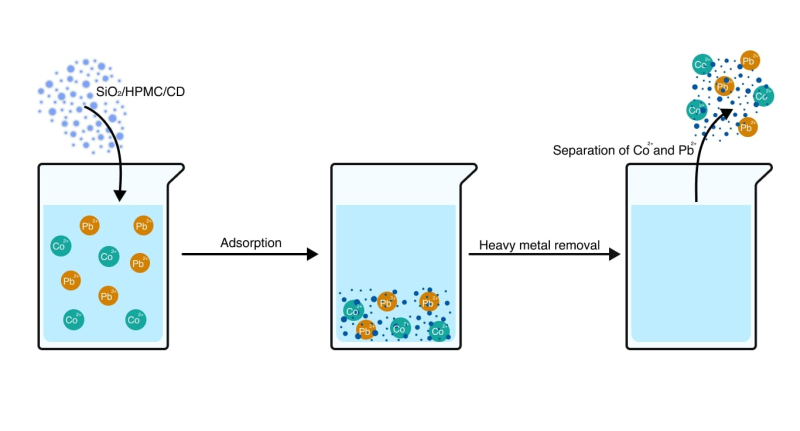
The sorbent works as follows: it needs to be immersed in polluted water where it adsorbs heavy metals on the surface and then extracted from the aquatic environment. Photo by the researchers
Another method in use is sorbents, both with wide applications and selective ones for separate metal ions. Due to ions’ selectivity, such sorbents can extract a specific metal ion from an aqueous environment. High sorption capacity allows for the use of a smaller mass of sorbent for cleaning to capture more pollutants on the surface. Also, if a sorbent can be easily regenerated, it can be used multiple times, and ions are efficiently converted into high-purity concentrated brines for their further processing, for example, in hydrometallurgy and catalyst chemistry. Nevertheless, the production of sorbents is a complex process that affects the environment, as well.
To that end, scientists from ITMO’s ChemBio Cluster developed a reusable, affordable, and efficient sorbent that removes ions of lead and cobalt from water.
“The sorbent is made up of two components, carbon dots and silicon dioxide. The latter has long been known as a porous, cheap, and easily-separated-from-water sorbent. Its downside, however, lies in the lack of functional groups, which negatively affects the sorption properties of silicon dioxide. Functional groups are found in carbon dots, also cost-efficient sorbents, but they are hard to remove from water and therefore can re-polliute it. That’s why we decided to combine both materials to reduce their negative features and synthesize a new hybrid sorbent,” says Egor Ryabchenko, the first author of the paper and a junior researcher at ITMO’s Laboratory of Applied Materials for the Energy Sector.

Egor Ryabchenko. Photo courtesy of the subject
Another advantage of carbon dots is their fluorescence; this can help researchers see how much metal is in the sorbent's active areas and when it needs to be replaced as the glow starts to fade.
The team ran an experiment to evaluate the porosity and surface area of the sorbent, as well as the amount of lead and cobalt removed in the presence of other metal ions, different contact time, temperature, acidity, and salinity. It was found that the sorbent has the maximum adsorption capacity of 258±4 mg/g and 168±6 mg/g for lead and cobalt, respectively. Under adverse conditions, such as high temperature and water acidity, the adsorption capacity of the sorbent decreased by half, yet it remained efficient.
The material is suitable for wastewater treatment at both productions and households. It works as follows: the sorbent needs to be immersed in polluted water where it adsorbs heavy metals on the surface and then extracted from the aquatic environment. Afterwards, it can be regenerated with a weak acid solution, which helps extract cobalt and lead salts. The efficacy of the material remains above 90% within three cycles of regeneration.
In the future, the team plans to experiment with other combinations of carbon dots and study how changes in synthesis conditions affect the final product in order to produce new materials for selective sorption of other metals.





