Hyaluronan
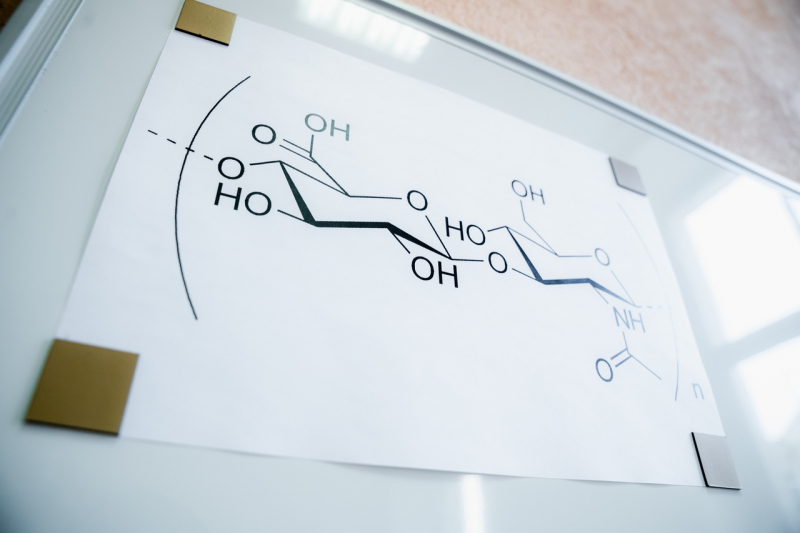
Hyaluronic acid is a natural linear polysaccharide that consists of periodically alternating acid residues of glucuronic acid and N-acetyl-D-glucosamine. The name stems from the Greek word hyalos, meaning glasslike, + uronic acid.
Actually, a more correct term would be hyaluronan, although most of the time we’re talking about one and the same material. It is a general name for a group of compounds which can be in the form of acids or salts. Any alternative names have to do with which compound properties researchers study or how they choose to apply them. For example, sodium hyaluronate is hyaluronic acid sodium salt. Hyaluronic acid is a naturally-occuring polymer that can be found in human and animal cells as well as the pericellular and intercellular spaces surrounding these cells.
Hyaluronic acid has a number of vital functions in living organisms: it participates in cell division, serves the process of nutrient transfer and metabolite extraction from cells, holds large quantities of water, takes part in healing processes, etc. As it is naturally-occuring, hyaluronic acid is a multipurpose, safe, and biocompatible substance.
What are its main sources?
Hyaluronic acid was first discovered and subtracted from vitreous bodies in cattle by Karl Meyer and John Palmer in 1934. Natural sources such as chicken caruncles, shark skin, and mammal umbilical cord still remain one of the main ways of hyaluronic acid subtraction. However, using acid obtained from tissues of living organisms can cause allergic reactions due to remaining endotoxins and protein components which could not be removed by purification. Another source is bacterial synthesis: certain strains of bacteria are grown in a substrate which creates conditions for biosynthesis of hyaluronic acid. From a scientific point of view, this method is more interesting because it allows acquiring hyaluronic acid in a wide range of molecular masses. We have to note that hyaluronic acid of animal origin has the largest molecular mass. We have to note, however, that only natural sources provide researchers with a large molecular mass of hyaluronic acid.

Why is molecular mass so important?
It determines the structural, biological, chemical, chemicophysical, and other properties of substances – and thus the possibilities and fields of their application. For instance, hyaluronic acid with a molecular weight of 20-200 kDa (kilodalton; dalton is a unit of mass used for molecules, atoms, atom nuclei, and elementary particles) takes part in processes of embryonic development, wound healing, and ovulation. On the other hand, hyaluronic acid with a higher molecular weight (over 500 kDa) can act as a natural immunosuppressant and has anti-inflammatory properties. Therapeutic properties of hyaluronic acid-based medical treatments also depend on its molecular mass. Its degradation rate, on the other hand, can determine the duration of action of a hyaluronic acid injection or the durability of prosthesis based on it. To fill the gap in the study of hyaluronic acid properties, Petr Snetkov with colleagues have published a review demonstrating the link between these properties and the polymer’s molecular mass.

Is hyaluronic acid really used in injections and prosthetics?
Let’s start with the basics. The presence of hyaluronic acid in many tissues and liquids determines its application in medicine and cosmetics. However, even in these fields it can be used in a wide variety of ways: from biorevitalizing cosmetics for skin and intra-articular liquid implants to polymer shells and healing wound coatings.
Thanks to the ability of hyaluronic acid to form lasting intermolecular and intramolecular hydrogen bonds, including those that link with water, polymer structures can absorb and retain water, which is used to manufacture moisturizing cosmetic products.

Not only injections
The prospects of hyaluronic acid applications are countless. It attracts chemists, biochemists, and bioengineers. Scientists think that it can be used to create artificial blood vessels and carcasses for growing cells, organs, and tissues.
Another application is wound and burn nanofiber coatings that can also speed up the healing of post-surgical sutures.
The acid can also help in fighting tumors. The latter have already been confirmed to be sensitive to polymer nanoparticles. Creating nanoparticles based on biocompatible and biodegradable hyaluronic acid can not only serve targeted drug delivery to affected organs, but also prolong the activity of delivered pharmaceutical substances.
Thanks to the polymer’s negative charge, there will be no lipidic shells that tend to clog blood vessels around it. It means that such treatments can be administered intravenously. Thus, nanofibers and nanoparticles are the most interesting, prospective, and topical field of research.

But that is not all there is!
Despite the fact that nanoparticles and nanofibers are obtained and studied in laboratories, there are a number of unsolved fundamental questions about the properties of hyaluronic acid.
This is a hydrophilic polymer, which means it only dissolves in water. If we add a co-solvent or non-organic salt to the solution, they can significantly change the polymer’s physical and physicochemical properties. The relevance of such fundamental research lies in the fact that drugs or cosmetic products contain not only the polymer itself but also other additives (preservatives, biologically active substances, etc.) which can influence the duration of the therapeutic effect.
Electrospinning (the method used to create nanofibers based on hyaluronic acid) has certain requirements for spinning solutions.
Aquatic solutions of hyaluronic acid are known to have a low solvent vaporization rate, as well as high viscosity and conductivity at low polymer concentrations, which hinders the spinning.
However, ITMO University researchers found a way to overcome this hindrance. They were the first to use dimethyl sulfoxide as a co-solvent – this is a widespread drug for external use. It not only made the obtained nanofibers absolutely safe for people but also added antiseptic and anaesthetic effects.
Such projects have a great potential for production of medical treatments with high efficiency, low level of systemic exposure, and targeted actionon affected organs – without any side effects.