FMT is now used to treat a wide range of conditions, especially infectious diseases. Moreover, many people turn to this procedure due to its preventive and paramedical properties. As is well known, microbiota have a profound influence on our physical and mental well-being.
From a biological standpoint, FMT also provides a unique chance for researchers to study the processes of intestinal colonization, as well as interactions between bacteria and their host organism.
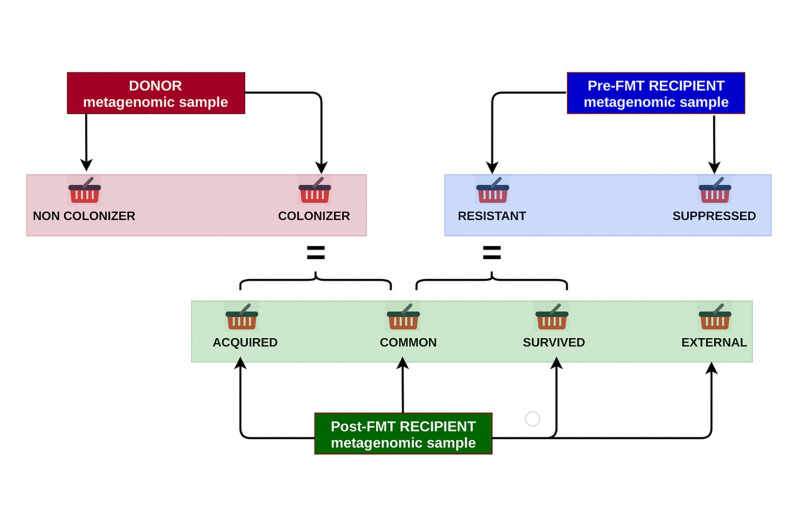
Scientists from ITMO’s Computer Technologies Lab took this challenge and decided to analyze a large amount of metagenomic data from the gut of microbiota donors and patients before and after the transplant using a specially designed RECAST algorithm.
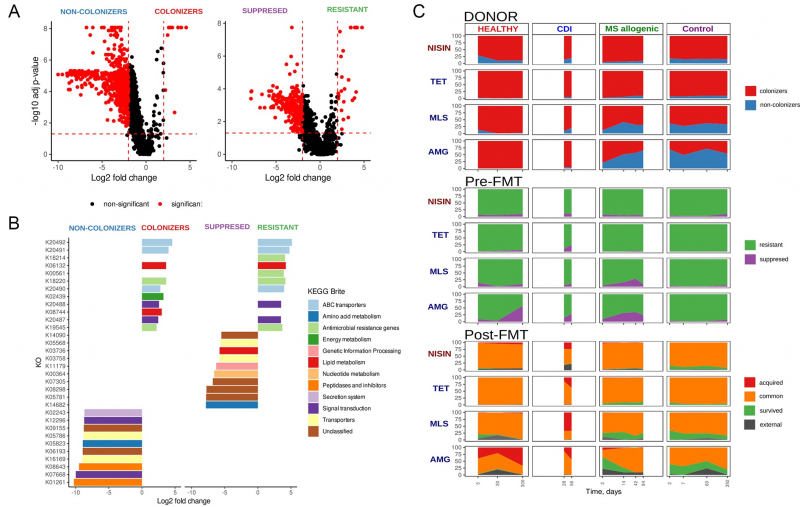
The researchers used data from healthy volunteers, patients with clostridial colitis, and patients with metabolic syndrome that helped them learn which donor bacteria can be successfully implanted and how they displace the recipient’s bacteria.
“We strived to figure out how the composition of donor and patient microflora affects transplantation. What defines which bacteria are here to stay? The research showed us that some bacteria can produce natural antibiotics, which allows them to compete with one another. As a result, the winners are those bacteria that are doing it better. Yet it also has something to do with the external response as bacteria of healthy people adapt to fight various pathogens inside the intestines,” says Artem Ivanov, a co-author of the paper and a PhD student at ITMO’s Faculty of Information Technologies and Programming.
In simple terms, the bacteria of a healthy person (a donor) are often much stronger than that of a patient with an intestinal disease. That is where the transplantation efficacy comes from. After all, the procedure significantly enriches bacterial composition in the gut, making a person less prone to infectious diseases. The possible reason is a larger number of bacteria that can produce antibiotics. And what’s more, the effect is observed in all patients, regardless of their diseases or physical condition.
A video summary: the insights and the findings of the study
Although microbiota are not always transplanted well, this problem can be solved through metagenomic studies. A more thorough selection of the donor in each and every case may vastly increase the efficiency of transplantation.
“We managed to identify the genes that can serve as our ‘weapons’ in the bacterial competition for resources. Our study produces one of the few models that illustrate what happens during transplantation at a genomic level. This can theoretically help us select the strongest bacteria for the procedure. Globally speaking, our goal is to learn how to manage the human microbiota. Surely, we are yet far from achieving this goal, however, our study is a stepping stone,” explains Vladimir Ulyantsev, a co-author of the paper and the head of ITMO’s Computer Technologies Lab.
As stressed by the researchers, the developed model should be further studied on a greater number of samples. In total, the current research was based on 223 real metagenomic samples from open international databases. However, here the scientists encountered a major problem: such data began to appear just a few years ago and on a small scale. That’s why scientists should conduct more similar studies in order to continue this work.
“This research provides, rather, fundamental conclusions, which should be further examined first and only then applied in personalized medicine. This approach will make it possible to select a specific donor or somehow modify donor samples so that they can be successfully implanted. So far, we’ve been working with what we have on hand. If we obtain more datasets and hospitals express their interest in our research, we will be able to start moving towards personalized medicine,” concludes Vladimir Ulyantsev.
Reference: Evgenii I. Olekhnovich, Artem B. Ivanov, Vladimir I. Ulyantsev, Elena N. Ilina. Separation of Donor and Recipient Microbial Diversity Allows Determination of Taxonomic and Functional Features of Gut Microbiota Restructuring following Fecal Transplantation, mSystems, Vol. 6, No. 4.