Porous materials are used in industrial chemistry for catalysis (accelerating chemical processes), chromatography (separating and analyzing mixtures), purification, and filtering systems. For their synthesis, scientists are looking for versatile reagents, namely inexpensive and easily produced substances that can be utilized to create materials with varied compositions and desired properties.
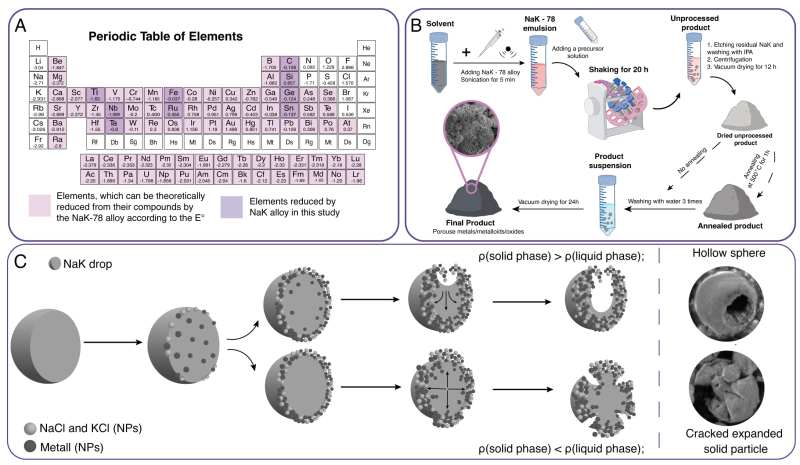
A liquid metal, a eutectic alloy of potassium and sodium NaK-78 with the melting point of -12.65 0C, was tested as a reagent by a team of chemists from ITMO and Skoltech. Using ultrasound, the team transformed the sodium-potassium alloy into an emulsion with an organic solvent, which they then combined with a precursor solution – a compound that participates in a reaction and produces a desired product (a metal or metalloid). This caused a reaction at the interface. As a result, the target material was recovered from the precursor (metal or metalloid chloride) in combination with by-products, which are easily removed with water and create holes in the structure of the final material.
Since the alloy is liquid, it can easily make an emulsion with other liquids – in this case, organic solvents. The emulsion consists of microdroplets with a large specific surface area, which accelerates the reaction between the liquid metal and the precursor solution. In addition, unlike other reagents, the metal allows for the different stages of the synthesis of porous materials to occur simultaneously: chemical reduction, as well as the formation of pores and material microstructures (morphological control).
Therefore, the synthesis of porous materials with the proposed method is easier and cheaper as chemists do not need additional reagents. Furthermore, the method is eco-friendly: potassium and sodium chlorides are non-toxic and can be washed away with water, which may then be disposed of with little treatment into the environment or separated into salts and water.
The method has already been proven solid in practice; the researchers applied the method to create porous materials based on ten chemical elements (carbon, silicon, germanium, tin, titanium, ruthenium, antimony, iron, niobium, and tantalum).
Additionally, the team has also demonstrated that the resulting materials can be used as components for energy storage systems such as sodium and potassium-ion batteries. In contrast to traditional lithium-ion, this type of battery is more affordable and eco-friendly and while it will not completely replace the former, it will be well-suited for large energy storage power stations and cars. In these batteries, the scientists employed porous tin and antimony manufactured using the novel technique as anode materials – electrodes where an oxidation reaction occurs.
Potentially, porous materials in such batteries can lessen the effect of anodic expansion, which happens during charging and causes anode damage, while also increasing a device's service life. It will also pave the way for more efficient industrial metal-ion batteries and catalysts and, therefore, obtaining more target substances through chemical reactions.

Sergei Leonchuk. Photo by Dmitry Grigoryev / ITMO.NEWS
“We want to expand our method to include more chemical elements, and we also intend to synthesize composite materials with specific compositions (for example, a carbon-based nanocomposite of porous tin instead of just porous tin). Composites play an essential role in the development of catalysts and batteries as they, on the one hand, provide high conductivity and on the other, protect materials from deterioration. We will be testing the materials developed jointly with our colleagues from ITMO’s Energy Lab and the Center for Energy Science and Technology (CEST) at Skoltech,” says Sergei Leonchuk, an author of the paper, a PhD student and an engineer at ITMO’s ChemBio Cluster.
Reference: Sergei Leonchuk, Aleksandra Falchevskaya, Polina Morozova, Nikolai Gromov, Vladimir Vinogradov. NaK alloy as a versatile reagent for template-free synthesis of porous metal- and metalloid-based nanostructures (Chemical Communications, 2024).