According to the WHO’s International Agency for Research on Cancer, 2022 saw 330,000 new registered cases of melanoma (a type of malignant skin cancer) and 60,000 deaths. Clinicians combine varied therapy methods to diagnose and treat the disease, including hyperthermia – localized heating of the tumor. This involves first introducing special materials – such as gold nanoparticles capable of transforming light energy into heat – into the tumor. This way, the temperature of the malignant growth is increased up to 40-50°C. The heating destroys protein structure, thus also destroying cancer cells.
During this process, it’s important to regulate the level of heating in real time, as temperature determines the expression mechanism of growth drivers and cell metabolism and death type. The latter is a predictor of whether cancer cells leave behind any products of degradation that can cause relapse.
Various approaches, including fluorescent quantum dots, Raman or scanning probe spectroscopy, can be used to regulate the heating. However, each of them has its drawbacks. It’s hard to adapt Raman spectroscopy and fluorescent methods for use in cells because of noise signals and the environment, while scanning probe spectroscopy can harm living cells.

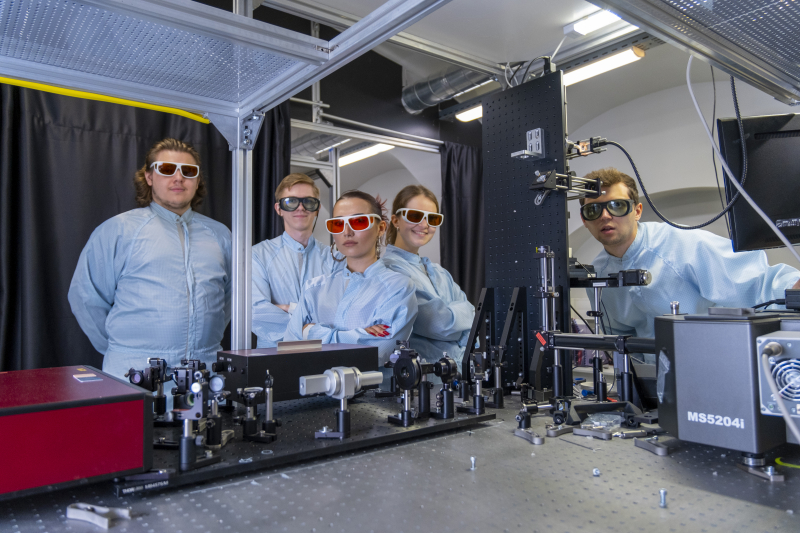
An installation for measuring optically detected magnetic resonance. Photo by Pavel Kiriltsev / ITMO's Faculty of Physics
An alternative for heat control can be nitrogen-vacancy nanodiamonds (materials with a disrupted crystal lattice where one of the carbon atoms is replaced by a nitrogen atom). Such nanodiamonds are often used in thermometry because of their biocompatibility, high sensitivity, and optical stability; they also do not photobleach or require additional calibration.
Scientists from ITMO were able to create a multifunctional nanomaterial that can perform two functions: locally heat the tumor and tissue like plasmonic nanoparticles and measure its temperature like a fluorescent nanodiamond.

The paper's authors (left to right): Ivan Vazhenin, Elena Gerasimova, Egor Uvarov, Vitaly Yaroshenko, Landysh Fatkhutdinova. Photo by Pavel Kiriltsev / ITMO's Faculty of Physics
The researchers suggest nanodiamonds with two types of coating: in one case, the surface is entirely coated in gold; in the other – a scatter of gold nanoparticles and a layer of silicon dioxide. The latter protects the nanodiamond from external charges and allows for more precision in thermometry.
This method is easy to use, as it requires only a single laser for heating and thermal measurements. With it, it’s also possible to grow gold nanoparticles to the needed size, thus regulating their heating capacity.
“We have tested the performance of plasmonic nanodiamonds in several experiments with a line of cancer cells and B16-F10 melanoma tissue outside a living organism. The higher the laser’s power, the lower the cells’ viability: the share of viable cells was 62.2% for nanodiamonds fully coated in gold and 51.32% – for gold nanodiamonds with a layer of silicon dioxide. We’ve also tested hybrid nanomaterials on four groups of mice with melanoma. The first one didn’t receive any treatment, the second was treated with a laser, the third – received plasmonic nanodiamonds, and the fourth – received nanomaterials and laser heating. Compared to control, the latter combination proved efficient: the malignant growth was stunted by 65.22%,” explains Elena Gerasimova, the paper’s first author and junior researcher at ITMO’s Faculty of Physics.
Elena Gerasimova. Photo by Pavel Kiriltsev / ITMO's Faculty of Physics
In the future, the new plasmonic nanodiamonds can be used for local melanoma therapy. For this purpose, scientists plan to investigate the photothermal process on a larger number of animals to understand which specific parameters influence the success of treatment, as well as to check how the activity of plasmonic nanodiamonds combines with another type of cancer therapy.
This research project is supported by the Russian Science Foundation grant No. 21-72-30018.