Sometimes, scientists make discoveries almost by accident: for example, when using structures intended for other experiments. The same happened when ITMO researchers managed to detect an unusual interaction between light and gallium-arsenide (GaAs) dimers – a pair of nanocylinders placed in close proximity to one another.
“Prof. Yuri Kivshar (a professor at the Australian National University and chief research associate at ITMO University – Ed.) contacted us and said that his team and their colleagues from Korea University had made samples to use in studying high-Q states within a single particle. However, when creating samples, they obtained not only the required single particles, but also dimers. And he suggested that we examine them to, perhaps, find something interesting,” recalls Mihail Petrov, the co-author of the article.
Mihail Petrov
Second-harmonic generation
Kristina Frizyuk, a PhD student at ITMO University, dealt with the theoretical aspect of the study. For a month, she studied the samples: nanocylinders made of gallium arsenide (GaAs), one the most commonly used semiconductor materials, and discovered unconventional properties of these particles associated with the second-harmonic generation.
“Second-harmonic generation is a nonlinear optical process used, for instance, in laser pointers,” explains Kristina Frizyuk. “An infrared laser with a wavelength of 1064 nm, when passing through a crystal without an inversion center, turns green and its wavelength changes to 532 nm. That is, its optical frequency doubles and wavelength is halved.”
Kristina Frizyuk
The same was true of the samples from Australia. When exposed to an infrared laser, they not only scattered the original light with a wavelength of 1,400 nm, but also emitted a visible red light with a wavelength of 700 nm.
The scientists used circularly polarized light, the kind commonly found in computer screens. This is the light that we can observe when a screen goes black if we use polarized glasses. While in the case of linear polarization the electric field vector always runs parallel to the chosen direction, in the case of circular polarization it moves in circles and has the same magnitude at each point in space. Photographers with autofocus cameras often have to deal with CPL filters turning incident light into circularly polarized light.
A GaAs crystal lattice. Illustration courtesy of the researchers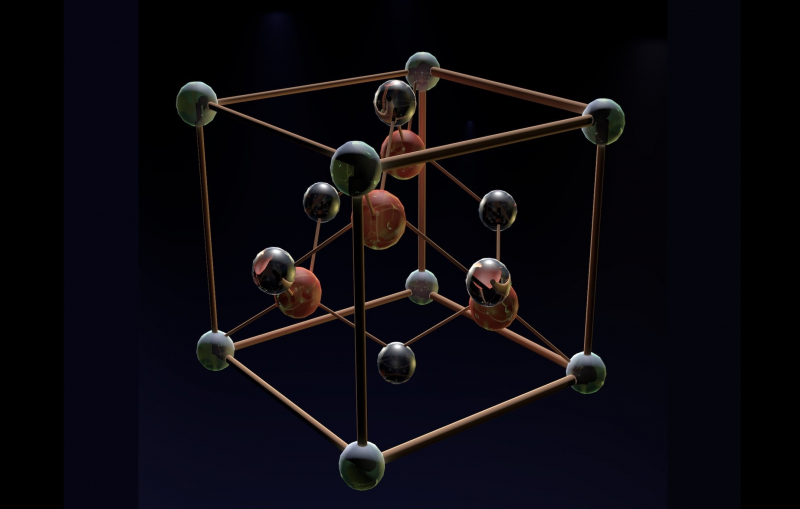
Irradiation of a single cylinder showed that the radiation pattern of the second harmonic has a complex shape that resembles a flower with four petals (four directions in which the light shines the strongest). This happens due to the symmetry of the crystal lattice. Changing the direction of the circular polarization (clockwise/counterclockwise) will result in the “flower” being reflected in a mirror plane while the total intensity of the second harmonic remains unchanged.
A graph showing the direction of light in cases of different circular polarization. Illustration from the article published by Nano Letters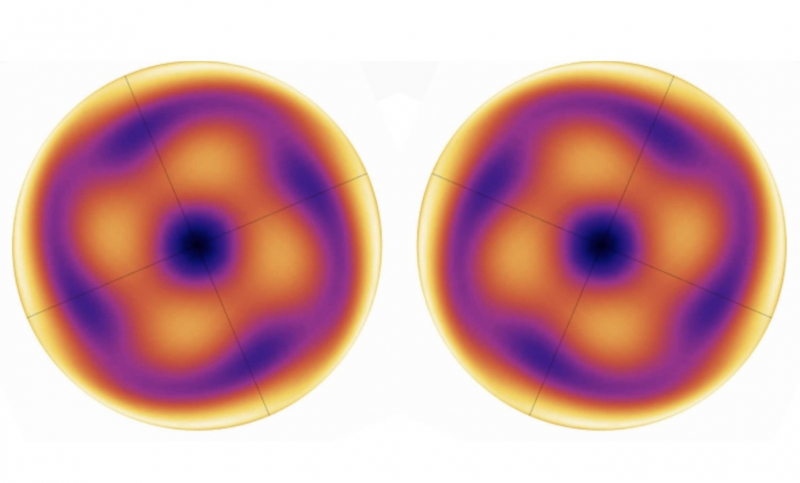
Dimers and flipped lattice
While conducting simulations, the researchers found that dimers significantly affect the difference in response to light rotating counterclockwise and clockwise. While the generation of the second harmonic will be strong in one case, it will be barely noticeable in the other. In other words, if you expose samples to infrared light with clockwise polarization, you will see a strong visible light generated by the dimer. In case of counterclockwise polarization, the generated light will be extremely weak. When confronted with this effect, the scientists were determined to find an explanation for the phenomenon.
Illustration courtesy of the researchers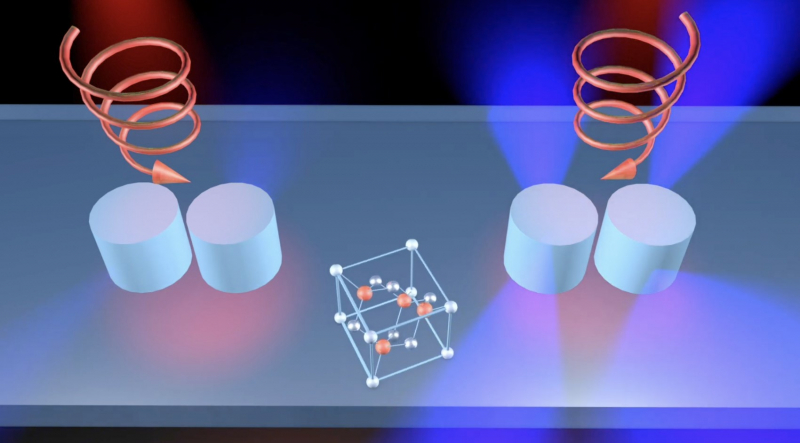
“We couldn’t understand even the simplest properties of such structures, so we started to study it deeper and found what we initially didn’t even look for,” explains Mihail Petrov.
As it turned out, the cause of nonlinear circular dichroism, as the scientists have dubbed the discovered effect, is a violation of internal symmetry during sample production. When the cylinders were transferred from one substrate to another, their position in space concerning their own crystal lattice became random. This experiment can be reproduced for simpler fabrication techniques, as well.
“When making such nanostructures, scientists usually use a relatively large monocrystal in which the crystal lattice is oriented in a certain way. You can carve or etch cylinders out of it at a direct angle or at any other angle towards the crystal lattice’s orientation. The latter case will result in an effect such as the one we’ve described,” explains Kristina Frizyuk.
The reason why these samples produce such a response can be found via symmetry analysis and examining the samples’ eigenmodes.
Nano Letters. Credit: pubs.acs.org
“It’s interesting that whenever we tried to somehow simplify the analysis or make a simple approximation, everything instantly fell apart and the effect went away. So it was important to look not at one, but at least two eigenmodes of a particle at once. In that sense, the theoretical part turned out to be more complicated than we had expected at first,” says Kristina.
Once the first theoretical results were produced, the researchers’ colleague from the Australian National University, Elizaveta Melik-Gaykazyan, who works under the supervision of Yuri Kivshar, conducted an experiment that fully confirmed their theoretical predictions.
Prospects
These findings will be beneficial for future research concerning other structures and materials. But the researchers’ discovery can have a practical application, too. Systems with such dimers can be used in special sensors capable of detecting so-called chiral molecules.
“The fact that we’ve got these structures capable of “telling apart” left and right polarization of light is very promising,” says Mihail Petrov. “The thing is, that’s exactly how chiral molecules behave. They’re these special molecules that don’t match their mirror image. These molecules can be very important, but working with them isn’t easy. Broadly speaking, a “left” molecule might be fit for medicinal use, but the “right” molecule might be a toxin. In the 60’s and the 70’s there was an issue with many innovative drugs in that they had severe side effects. It turned out that the reason for this was that the manufacturers didn’t completely separate the “left” molecules from the “right” ones. Even the well-known drug ibuprofen is, in fact, made up of a 50:50 mixture of “left” and “right” molecules, the activity level and qualities of which, however, differ greatly.”
The researchers are currently continuing their work on the discovered effect with the aim of better understanding how to use the properties of dimers to detect such molecules.