“Nowadays, kidney stones may go undetected till fairly late stages because of the untimely diagnosis of the disease and many other reasons, as well. To avoid that, we’ve designed a sensor specifically for uric acid. The device’s technology stems from our experiments with sensors for tick-borne encephalitis virus (TBEV), SARS-CoV-2, as well as certain antibiotics and microelements. Based on tailor-made electrodes made of polymers and sensitive components, the sensor delivers results in several minutes,” comments Ulyana Noskova, a co-author of the paper and a second-year Bachelor’s student at the Infochemistry Scientific Center who started conducting the study when she was still at school.
Fast, accurate, and easy-to-use, the novel sensor is suitable for running tests not only at medical laboratories but also at educational organizations and companies to monitor the health of students and employees, as well as for in-home usage.

Ulyana Noskova. Photo courtesy of ITMO’s Infochemistry Scientific Center
Inside the sensor
The sensor consists from several components: electrodes made of carbon fiber and an oxidation-reduction mediator; and a thin polyelectrolyte film, the molecular groups of which can become negatively or positively charged in a solution. The urate oxidase, settled between the layers of polyelectrolyte, is responsible for detecting uric acid in a biomaterial, whereas the charged polyelectrolytes can immobilize the urate oxidase as a magnet to make it catch uric acid molecules.
“We struggled to pick the polyelectrolytes so we had to thoroughly study how they affect the performance of our sensor,” explains Anna Baldina, a PhD student and a senior researcher at the Infochemistry Scientific Center.

Anna Baldina. Photo courtesy of ITMO’s Infochemistry Scientific Center
How it works
The sensing principle of the sensor is built upon a reaction between uric acid and an enzyme, namely the urate oxidase. Putting the sensor into a liquid, which allegedly contains acid, triggers the acid’s interaction with the urate oxidase, which results in a change of the electrical potentials in the substance. The signal is then sent to a computer via carbon fiber and is analyzed by a well-trained neural network to detect the percentage of the urate oxidase that has reacted – or, simply put, the level of uric acid.
“This research is part of a huge effort to create a versatile sensor platform to diagnose a range of biomarkers. We are still trying to figure out how various polyelectrolytes, active substances, and their mixes make it possible to analyze biofluids, such as sweat, saliva, urine, and blood,” adds Anna Baldina.
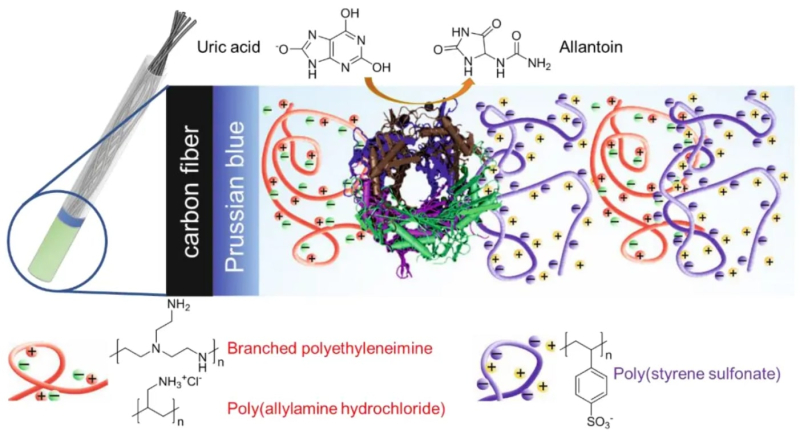
A setup of the device. The carbon fiber is coated with the Prussian blue mediator and layers of polymers (polyelectrolytes). The urate oxidase settles between two layers and detects molecules of uric acid – the marker of kidney stones. Illustrations from the article.
The experimental part of the study was mostly performed by Liubov Pershina, a student of the Infochemistry Master’s program, who recreated the assembly of carbon fiber and Prussian blue mediator modified with layers of polyelectrolyte and with urate oxidase in between, as well as analyzed sensor readings from various polymers. The study employed the quartz crystal microbalance (QCM) method, which allows researchers to monitor small mass changes of the sensor following a change in its composition due to the adsorption of biomolecules, be it antibodies, antigens, or enzymes, as in the case of the urate oxidase. Since the microbalance can operate not only in air or vacuum but also in a liquid, it is widely common in biochemistry.
Reference: Baldina, A.A., Pershina, L.V., Noskova, U.V., Nikitina, A.A., Muravev, A.A., Skorb, E.V., Nikolaev, K.G. Uricase Crowding via Polyelectrolyte Layers Coacervation for Carbon Fiber-Based Electrochemical Detection of Uric Acid. Polymers, 2022.
Written by Tamara Besedina and Maksim Sannikov