Atrial fibrillation is the most common cardiac arrhythmia that affects up to 2% of the population. The condition is caused by the abnormal growth of connective tissue and the formation of cicatricial changes, typically as a result of chronic inflammation. Due to that, patients experience rapid and irregular heartbeats, usually more than 400 beats per minute. The disease can lead to blood clots and increase the risk of stroke, heart failure, and other conditions.
To reduce the risk of developing heart-related complications, patients are generally prescribed antiarrhythmics, anticoagulants, or other medications, which are, unfortunately, not guaranteed to eliminate all the symptoms. In this scenario, specialists recommend catheter ablation – an invasive procedure that guides a tube into a patient’s heart to isolate a pulmonary vein and destroy the tissues responsible for abnormal heartbeat.
Nevertheless, the recurrence rate with such treatment varies between 20-45%. If atrial fibrillation does recur after the surgery, patients need to repeat the procedure. The main cause of recurrence is that the standard surgical protocol contains no predictive systems to evaluate the effectiveness of an inflicted “scar.”
The outcomes can be improved with a personalized protocol, tailored to each patient’s individual characteristics: heart structure, tissue growth, and so on.
So far, there exists only one system that can generate custom protocols for catheter ablation – OPTIMA (developed at Johns Hopkins University). The system generates a 3D simulation of a heart based on MRI to predict treatment outcomes. To date, OPTIMA reduces the risk of recurrence by 30%.
As stated by Valeriya Tsvelaya, a principal investigator (PI) at ITMO’s Laboratory for Experimental and Computer Cardiology (part of the Advanced Engineering School), this method, built on an electrophysical model, fails to account for the morphological characteristics of cardiac cells (their shape, size, etc.) that drastically impact the occurrence of arrhythmia – and therefore the shape of the “scar.”
The new solution
In order to prevent surgical complications, researchers from ITMO’s Advanced Engineering School, as well as their colleagues from Almetyevsk State Oil Institute, the company Tatneft, Moscow Institute of Physics and Technology (MIPT), Moscow Regional Clinical Research Institute named after M.F. Vladimirsky (MONIKI), and the Central Clinical Hospital of the Administrative Directorate of the President of the Russian Federation, suggested using simulation modeling to produce personalized protocols for surgeries. The developed AI-powered algorithm reconstructs atrial conduction, meaning that it locates heart rhythm disturbances, based on cardiac MRI. The scientists believe the developed model will reduce the recurrence rate by over 30%.
The method draws on a cellular model that detects fibrosis – areas of the heart with fibroblasts, also known as cells that do not carry impulses to the heart and disrupt its operation – to produce a surgical protocol for each patient. The team expects the cellular model to be more sensitive to data on a patient's fibrosis, as opposed to an electrophysiological one. Given the personalized approach, the technology is to make procedures more efficient.
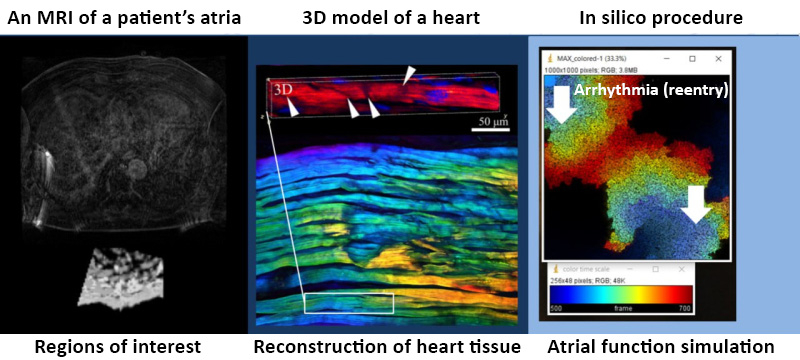
There are three stages in the process. First, the neural network segments MRI data and determines the structure of the atria to separate fibrous and healthy cells. Afterward, the algorithm distributes the cells in accordance with the fibrosis pattern utilizing the method of parameterization and creates a digital twin of the heart. Finally, it models several surgical protocols from which medical specialists can select the best. Credit: AIP Publishing
To create the model, the specialists used around 70 cardiac MRIs (more than 300 scans in different planes) before and after catheter ablation from open-source databases.
What’s next
The team has already designed a prototype of the neural network for segmenting atrial walls and plans to go further with their project. The developed method will pave the way for decision support systems for cardiac surgeons if more labeled MRI scans are obtained. The system will predict the most suitable protocol for each patient – the one that will ensure a lower chance of recurrence after the surgery.
“We’ve already defined cell parameters for cellular models, collected a portion of data on cell bioelectric potentials in patients of different ages, started to train an algorithm on this data, and produced a test version of our neural network that can identify the shape of atria and fibrous accumulations through an MRI scan. Later on, we plan to put all of our findings into one system: collect more data, complete the training, load the data into one program, and run several comparison tests against existing analogs,” shared Valeriya Tsvelaya, the project’s leader and PI at ITMO’s Laboratory for Experimental and Computer Cardiology.