Today, more and more attention is paid to the quality of air and water and control of any harmful compounds they may contain. Even a small concentration of such compounds can have a tremendous negative effect on the health of humans and animals. Moreover, these days chemists, physicists and materials scientists experiment with extremely small quantities of various substances in small concentrations, and that’s why they need to have a reliable way to determine even the slightest amount of any impurities in the substance.
We need complex equipment to monitor the chemical composition of substances and identify specific compounds. The most widespread of the previously applied methods is vibrational spectroscopy.
“With vibrational spectroscopy, you can easily learn the molecular composition of any substance previously unknown to you,” explains Daler Dadadzhanov, PhD student in a joint program of ITMO University and Ben-Gurion University of the Negev, Israel, research associate at the International Research and Education Center for Physics of Nanostructures. “It works like this – we have an unknown substance that consists of a number of atoms interacting with each other; an amino group, for example, has hydrogen and nitrogen atoms. When subjected to light radiation, these atoms start oscillating, absorbing a certain amount of energy while they’re at it. As a result, the outcoming energy will be less. The frequency at which the energy was absorbed, can be used to determine the functional atomic groups a molecule consists of. Then, a ‘molecular ID’ could be created that can then be used by a detector as it determines the kind of substance it was presented with.”

The spectrometers used today usually operate in the mid-infrared spectral range, with the wavelength of 2.5-25 micrometers. In this range, the differences between the energy of incident light and the energy that already passed through the substance can be easily defined and analyzed. The analyzers working in this range, however, are relatively large and cumbersome, as well as rather expensive. Moreover, some bands in the mid-infrared spectrum are so intense, like those connected to hydrogen atoms’ vibrations of an OH group, that they lead to total energy absorption when detecting small quantities of substances. These bands are the cause of difficulties when interpreting other characteristic vibrational bands in the absorption spectrum.
Bringing the spectrum nearer
The system could be made several times smaller, if it could operate not in the mid-infrared but in the near-infrared spectrum consistent with short-wavelength radiation. The near-infrared spectrum is studied much more than the mid-infrared one – mostly because it is used by modern telecom systems.
“The main advantage of the near-infrared spectrum is that nowadays there are a lot of energy efficient and high-quality continuous radiation units and reliable detectors,” comments Daler Dadadzhanov. “They are cheaper than those used in the mid-infrared range and more compact. Thus, the mid-infrared spectrum equipment can be 1.5 by 1.5 meters in size, while the near-infrared one could fit on a human palm.”
However, there is a problem – making the wavelength shorter means that the difference between the incoming and outgoing energy becomes too small to be easily detected. As a result, a bigger amount of substance is needed for good-quality analysis, which puts compacting the device at risk. Furthermore, many sensors are aimed at detecting unknown substances with marginally small concentrations, such as toxic molecules. The task becomes harder in the near-infrared spectrum.
Before creating an analyzer based on near-infrared vibrational spectroscopy, scientists need to find a way to amplify the signal received because of the difference between the incoming and outgoing energy. This was what the researchers from Ben-Gurion University of the Negev, Israel headed by Dr. Alina Karabchevsky and their colleagues from ITMO University were working on.
Gold can help
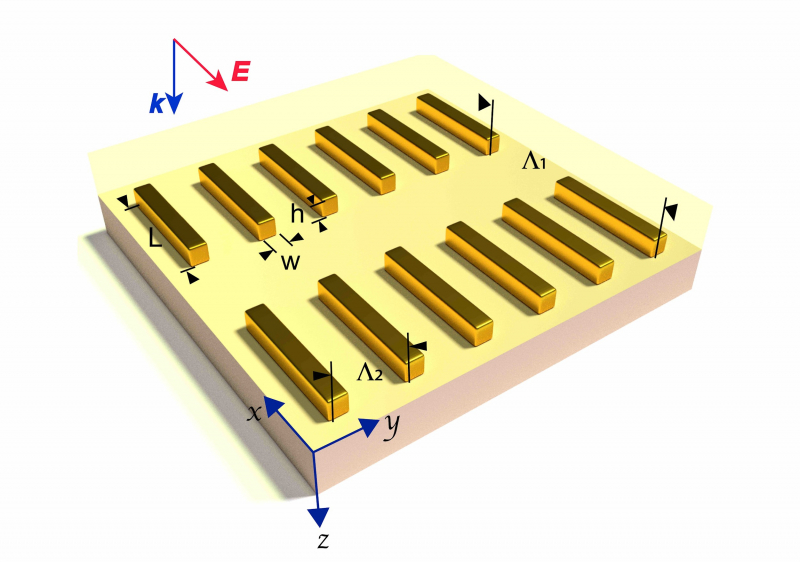
At ITMO, researchers have long been synthesizing metal (plasmonic) nanoparticles and studying their photophysical properties. It is these nanoparticles that it was proposed to use to amplify the signal for vibrational spectroscopy. Despite the fact that the plasmonic nanoparticles have been studied for a long time, until recently they haven’t received a lot of attention from researchers.
“In our paper, we propose the following design – on a base of a transparent dielectric, like, for example, borosilicate glass, a periodic array of gold nanoparallelepipeds is formed. Such structures can be acquired with electron-beam lithography,” continues Daler Dadadzhanov. “After that, we cover the substrate with a thin layer of the studied substance and register the transmittance spectrum of the sample, which is conditioned by combined excitation of plasmonic resonance in gold nanoparticles and molecular vibrations (overtones) of the studied substance. Gold nanoparallelepipeds with the proposed form have their plasmonic resonance in precisely the same range of the spectrum where the studied molecules have their absorption bands. Moreover, in the vicinity of a metal surface the electromagnetic field is strongly amplified. Therefore, it increases the proposed sensor’s sensitivity.”
The paper published is theoretical – with research conducted on numerical models. The next stage, therefore, will be to conduct actual experiments in creating such systems under laboratory conditions.
Reference: Daler R. Dadadzhanov, Tigran A. Vartanyan, Alina Karabchevsky. Lattice Rayleigh Anomaly Associated Enhancement of NH and CH Stretching Modes on Gold Metasurfaces for Overtone Detection. Nanomaterials, 2020.