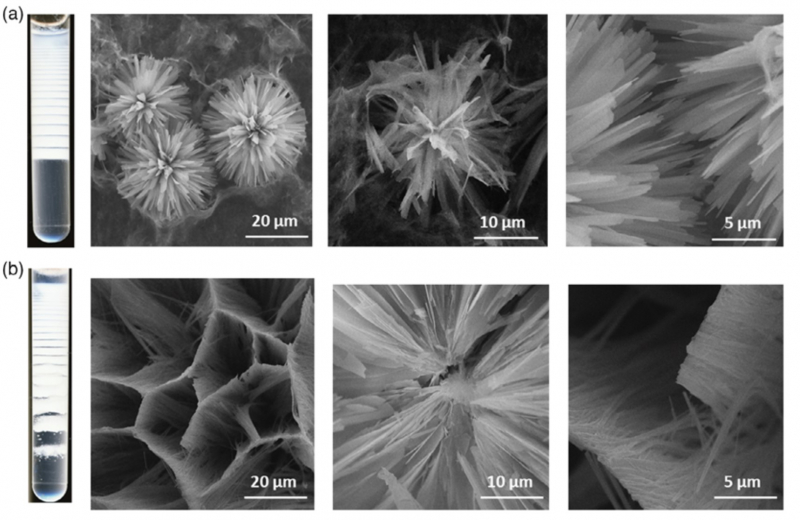
Despite the constant study of human biology, many processes of our bodies remain under-researched. In particular, it’s still not entirely clear how different amino acids and other substances influence the growth and healing of bones.
“Scientists today are eager to understand how amino acids, hormones, and vitamins influence different processes that take place in our bodies and tissues,” says Svetlana Ulasevich, an associate professor and researcher at ITMO University. “There is a hypothesis that amino acids can facilitate the biomineralization and stabilization of hydroxyapatite – calcium phosphate that forms our bones.”

Cellular incubator
It’s extremely hard to study this process in vivo, i.e. directly in the human body. That’s why it’s important to have a lab model – an environment with physical properties similar to those of living tissues. Scientists from ITMO University have developed a structure that resembles the human body in terms of resilience and acid-base balance.
“We proposed a model system for studying the process of bone formation,” adds Svetlana Ulasevich. “In our bodies, bones form in an organic matrix that is mostly made up of collagen, gelatin, and surrounding tissues. In the lab, we also form a matrix out of agar – a plant-based gelatin substitute. In this environment, we also form calcium phosphates and study how and why they develop. As a result, we end up with structures that have different properties. Then, cell cultures are planted onto this material and we can observe the way bone tissue cells grow.”
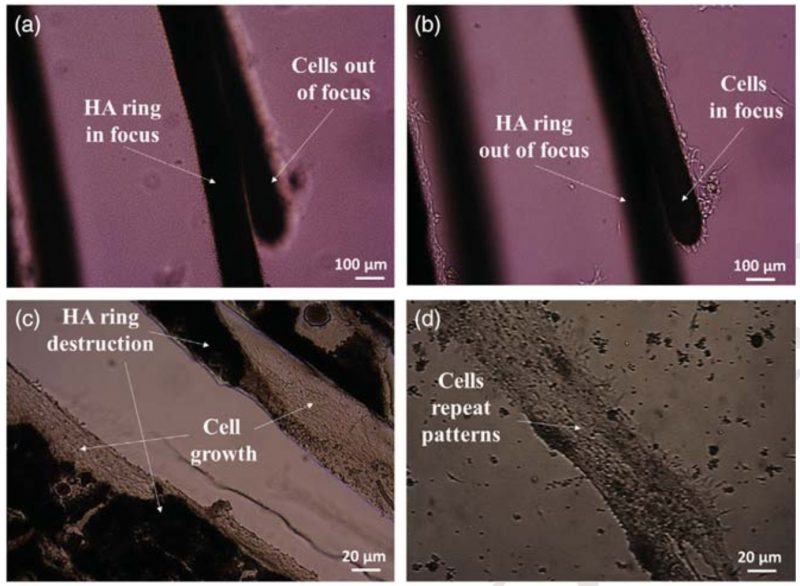
The first experiments were conducted using C2C12 cells of muscle tissues. These cells can differentiate – they turn either into bone cells or become a part of muscle tissue, depending on the body’s needs.
Fundamental and applied uses
This model can be used for two tasks. Firstly, to conduct fundamental research in the field of biochemistry of cell growth. This is needed in order to better understand how bones grow, why failures might occur in this process, and how to prevent related diseases.
“If we are to understand how bones are formed and how this or that molecule influences the behavior of our cells, then we will be able to come up with new ways of treatment and prevention of different diseases, such as osteoporosis and osteomyelitis,” explains Svetlana Ulasevich.

Secondly, these model systems can be applied in the creation of cellular structures. This might be useful for 3D bioprinting and for the creation of structures with different properties as part of one sample.
“We have detected an interesting phenomenon as we were studying the growth of cells on preliminarily formed patterns of calcium phosphate,” explains Svetlana Ulasevich. “Our model system has a pattern made out of concentric circles and cells prefer to grow on these circles specifically. We plant cellular structures over the entire area but tissues form on the circles primarily. This means that our material allows us to create cellular patterns of any form we need. In the future, this might become a basis for smart wound-healing surfaces that will facilitate healing of tissues in specific places.”
The study was published in the journal Advanced NanoBiomed Research.
Reference: Mervat M. Eltantawy, Mikhail A. Belokon, Elena V. Belogub, Olesia I. Ledovich, Ekaterina V. Skorb, and Sviatlana A. Ulasevich. Self-Assembled Liesegang Rings of Hydroxyapatite for Cell Culturing. Advanced NanoBiomed Research, 2021/10.1002/anbr.202000048