In 2020, cancer accounted for nearly 10 million deaths globally, making it a leading cause of death worldwide. Among the methods used to treat and diagnose cancer are MRI scanning and hyperthermia, a kind of therapy where tumors are heated up to 41-45°С before proceeding with radio- or chemotherapy. Hyperthermia increases the efficiency of other therapy methods.
Both MRI scanning and hyperthermia may rely on magnetic nanoparticles used in these cases as a contrasting agent, helping detect the tumor.
In hyperthermia, nanoparticles are actually the ones destroying cancer cells – when subjected to a magnetic field, the particles heat up, thus breaking the tumor apart. Recently, a research team from ITMO has suggested a magnetic particle from zinc ferrite and manganese ferrite that can be used both in cancer diagnostics and therapy.
Such particles are challenging to synthesize because a number of properties has to be considered in the process, including their size, shape, compounds, and coating. Conventionally, these particles are synthesized and then tested manually, which is a lengthy process – a single particle takes 4-6 hours to synthesize.
That is why chemists around the globe are looking to systematize information about existing particles, by, for instance, analyzing the efficiency of previously synthesized nanomaterials and their characteristics. However, these are related in a complex way: the efficiency of particles is determined by many characteristics at once, so it is rather difficult to combine them within a single formula.
A solution lies in using machine learning methods, which are already helping researchers predict nanomaterials’ drug delivery properties, automate experiments, and synthesize chemical compounds with specific properties. Such methods have not previously been used to synthesize nanoparticles used in MRI scanning and hyperthermia.
Read also:
IT and Chemistry for New Medicine: ITMO Hosts Digital Pharmacology Hackathon
A solution from ITMO
A group of researchers from ITMO became the first to suggest using machine learning to predict the efficiency of magnetic nanoparticles used for these purposes. They developed an algorithm that receives nanoparticle properties as input – and as an output describes their efficiency in cancer treatment and diagnostics. This is accomplished within a few seconds.
The algorithm was trained on open data about 980+ magnetic nanoparticles obtained during in vitro experiments. At the core of the algorithm are ensemble models based on decision trees: LGBM Regressor and ExtraTrees Regressor.
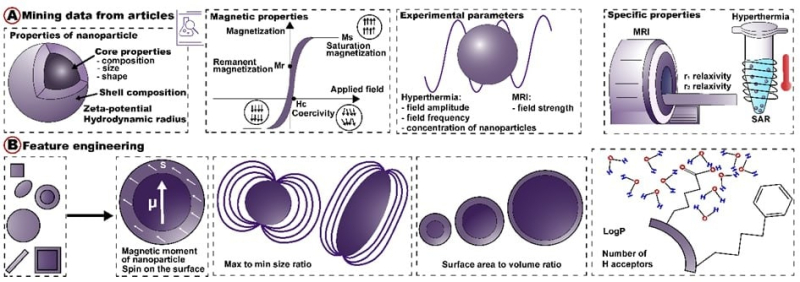
As its input, the algorithm receives data on particles’ size, shape, surface, compounds, and electromagnetic field (image A). Additionally, the algorithm calculates new parameters to improve the accuracy of its prediction (image B). As its output, the algorithm describes nanoparticles’ efficiency with the following metrics: r1/r2 relaxivities in MRI and specific absorption rate (SAR) for hyperthermia. Image courtesy of the authors
When testing the algorithm, the researchers measured its efficiency with RMSE, a metric that demonstrates how much the predicted properties differ from the actual ones. The smaller the number, the more accurate the algorithm is. The model was trained to predict specific absorption rate (SAR), r1 and r2 relaxivities (the metrics crucial for its applications), and the results are respectively: 0.26; 0.27; and 0.22. These values are considered minimal and signify the algorithm’s high precision.
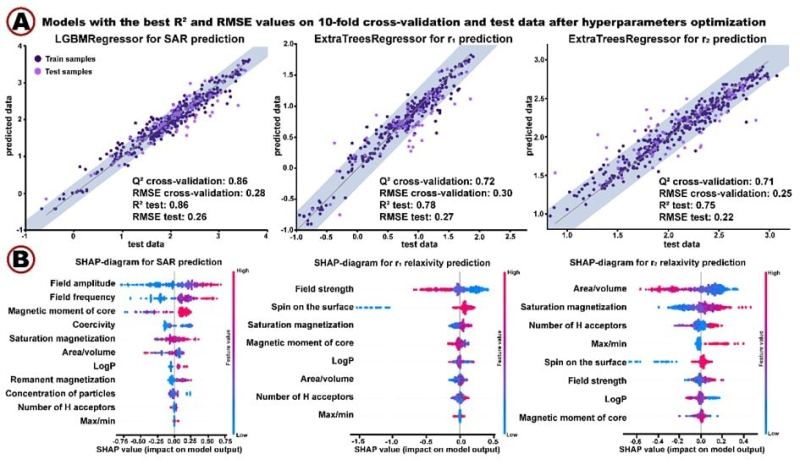
Results of the algorithms’ application. (A): Three graphs, each demonstrating the accuracy of efficiency predictions. (B): Interpretation of machine learning models via SHAP diagrams. Image courtesy of the authors
What’s next
Apart from the algorithm, the researchers have also developed DiMag, an open-source online resource that helps scientists choose the right properties of their nanoparticles during synthesis to achieve a specific level of efficiency. The service can also visualize dependencies between particle properties. Moreover, users can upload their experimental data from studies with nanoparticles.
“Currently, the algorithm has been trained on in vitro data, which is the first step in selecting high-efficiency particles for MRI diagnosis and hyperthermia treatment of cancer. Next, we are planning to train it on in vivo data. This way, we will be able to test medicines based on synthesized nanoparticles on live organisms – and later introduce them in clinical practice,” shared Pavel Kim, one of the authors of the paper and a Master’s student at ITMO’s ChemBio Cluster.
This research initiative is supported by the national program Priority 2030.
Reference: Pavel Kim, Nikita Serov, Aleksandra Falchevskaya, Ilia Shabalkin, Andrei Dmitrenko, Daniil Kladko, Vladimir Vinogradov. Quantifying the Efficacy of Magnetic Nanoparticles for MRI and Hyperthermia Applications via Machine Learning Methods (Small, 2023)