Genetics and autoimmune diseases
There are about 140 identified autoimmune diseases, including psoriasis, first type diabetes, rheumatoid arthritis, and Crohn’s disease. All of them cause the body’s immune system, its antibodies, to turn upon itself, and most of them are incurable but with possibilities to achieve remission.
Both autoimmune diseases and allergies are connected to changes in gene regulation. One way to identify the gene loci responsible for regulation and development of various diseases is through mapping. In the process, researchers end up with data about an extended section of the genome with dozens of genes that can have thousands of genetic mutations. Therefore, at this stage of mapping, researchers can only identify wide genome regions that contain the coveted mutations (variants). Moreover, the existing mapping methods are based on chip data obtained when the genome isn’t read but rather coated with markers every 6,000 nucleotides. With this method, only low-resolution mapping can be performed that cannot account for the whole diversity of nucleotide mutations in the genome.
In order to move further and detect mutations in specific genes, researchers from ITMO in collaboration with their Estonian colleagues have conducted fine-mapping of genetic factors inside the genome’s loci using the fully sequenced genomes from the Estonian Biobank (University of Tartu).
“We have decided to approach autoimmune diseases from the point of view of evolutionary genomics, hypothesizing that part of the genetic mutations behind these diseases are actually evolutionary adaptations. They have presumably remained in the immune system in order to help protect the human body from pathogens. Earlier the genetic changes behind these adaptations helped us survive, but now, in our world of antibiotics and hospitals, they show their dark side, contributing to the development of allergies and autoimmune diseases. There is a hypothesis in biology that evolutionary changes occurred only to allow organisms to survive and procreate, while any further “side effects” like sclerosis, diabetes, or psoriasis don’t matter in an evolutionary perspective,” explains Bayazit Yunusbayev, the paper’s co-author and the head of SCAMT’s Evolutionary Biomedicine lab.

Bayazit Yunusbayev. Photo by Dmitry Grigoryev, ITMO.NEWS
The paper’s authors turned to bioinformatics to find the specific nucleotide changes responsible for autoimmune diseases in the previously identified regions: they modelled and reconstructed those evolutionary changes in the human genome on supercomputers. In their analysis, the scientists relied on Relate, an approach that reconstructs the genealogy of small genome regions with candidate risk single-nucleotide polymorphisms (SNPs). Having completed the genealogy reconstruction, the researchers used CLUE, an algorithm that calculates the probability of neutral and adaptive evolution, identifying natural selection traces in a genome region.
Results of the study
In total, the scientists analyzed a database with over 2,300 sequenced genomes, identifying 535 genome loci connected to 21 autoimmune diseases and allergic pathologies, including diabetes, asthma, arthritis, psoriasis, and others.
About a third (204) of these risk loci were affected by natural selection, while in 40% of cases the most probable nucleotide mutations connected to a pathology were most likely a target of natural selection. In the future, these loci can be used for functional analysis (experiments with tissues and cells) that will reveal what these mutations are responsible for.
Moreover, the researchers have put forward a hypothesis explaining the activation of genes in the immune system that are responsible for autoimmune diseases. It is established that over 90% of the supposed genetic mutations are regulatory, meaning that they do not impact the protein structure but are activated in reaction to an outside stimulus. According to the evolutionary hypothesis, the genetic variants identified by the scientists most likely activate the genes in response to viruses and bacteria.
Last but not least, the scientists identified the cells and tissues in the immune system where the discovered nucleotide variants activate their functions. For instance, in case of autoimmune thyroiditis it’s B lymphocytes, T lymphocytes, and regulatory T lymphocytes, on the surface of which there is an increase in FCRL3 receptor synthesis. This receptor is responsible for intensified pathogen detection in the mucus membrane of lungs and intestines. In terms of evolution, this was a beneficial mechanism that protected our bodies from lethal pathogens, but in our time it increases reactivity to symbiontic bacteria in the intestines, thus contributing to the development of autoimmune thyroiditis. The data produced by the researchers will solve the second significant issue in genomics by pinpointing the cellular system for further experiments.
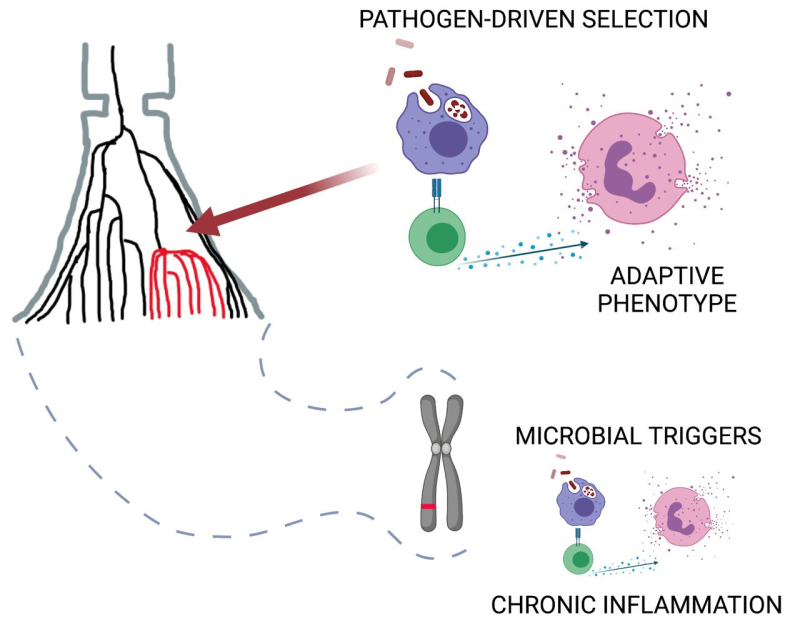
A schematic of selection of immune system adaptations to pathogens. It is suggested that these adaptive mechanisms are triggered in response to infectious triggers of autoimmune and allergic diseases. Image courtesy of Bayazit Yunusbayev
What next
This study can bring about a new approach to treating autoimmune and allergic diseases, which these days are treated with therapeutic monoclonal antibodies that selectively block parts of the immune response. One side effect of such medications, however, is that they can also hamper those immune reactions that protect the body from dangerous infections, such as tuberculosis. Moreover, a patient’s immune system can develop resistance to treatment, rendering it non-efficient.
According to the findings in this study, autoimmune diseases require comprehensive therapy that must include suppression of those microorganisms that trigger chronic viral and bacterial infections (i.e., tonsillitis, periodontitis, etc.). Such new antibacterial methods that will remove the factors triggering chronic infections will alleviate the symptoms of autoimmune and allergic pathologies.
The next step of the study is conducting experiments to test these hypotheses – for instance, by analyzing how specific viruses and bacteria affect the regulation of genes connected to autoimmune and allergic diseases. In particular, the researchers are planning to see how gene regulation changes in response to infectious triggers in the cells of patients’ immune system.
Reference: Vasili S. Pankratov, Milyausha M. Yunusbaeva, Sergei S. Ryakhovsky, Maksym Zarodniuk, Estonian Biobank Research Team & Bayazit B. Yunusbayev. Prioritizing autoimmunity risk variants for functional analyses by fine-mapping mutations under natural selection (Nature Communications, 2022).





